Introduction
This paper describes an innovation process design for a pharmaceutical manufacturing innovation project which intends to transform the supply of generic medicines to allow a sustainable, eco-friendly, and decentralized manufacturing. Research performed on intra-organizational processes, clusters of innovation, and customer engagement in new product design provided the theoretical framework for this innovation process design. The product of this paper will be part of an NSF grant request on Future Manufacturing (NSF, 2020).
Humanity’s needs for sustainable medicines supply – why we need it?
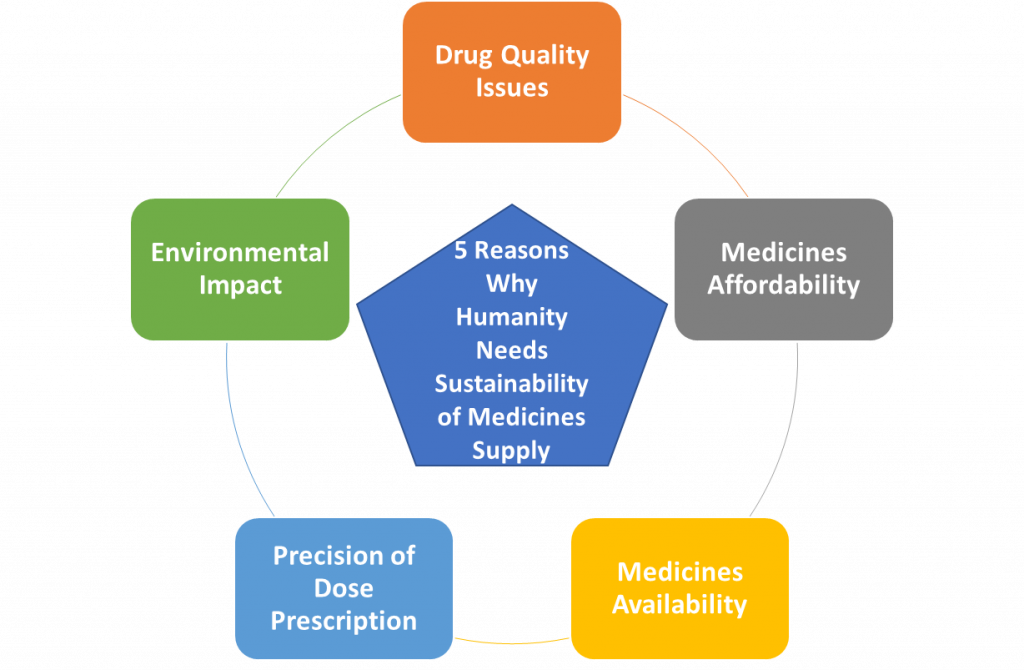
Humanity needs a dramatic change in the way we make and deliver medicines to patients. There are many “pains” that call for a dramatic shift in the way patients have access to drugs. Issues like drug quality, medicines affordability, medicine availability, the precision of drug dose prescription and the environmental impact of drug manufacturing and supply channels are preeminent “patient’s pains” and reasons why humanity needs such change.
The making of medicines had not changed much in almost 70 years. Manufacturing technologies developed in the 1950s are used in today’s pharmaceutical companies (Chaterhee, 2012). Figure 1 shows a comparison of a 1960’s rotary tablet press with a current-day tablet press. Other than a beautiful stainless-steel enclosure, the internal mechanism and design of both tablet presses follow the same principles. Technology changes have been historically slow in this regulated industry, but there is hope that is changing (Gottlieb & Woodcock, 2019, Hausner &Moore, 2018, National Science Foundation, 2006, and Pharmaceutical Technology Editors, 2016). The pharmaceutical industry is currently pursuing modernization of medicines manufacturing with the support of government regulators to change the way drugs are produced at the industrial setting, and with the hope to impact drug manufacturing quality and availability positively by the avoidance of drug shortages (Gottlieb & Woodcock, 2019, and US GAO Report, 2016).
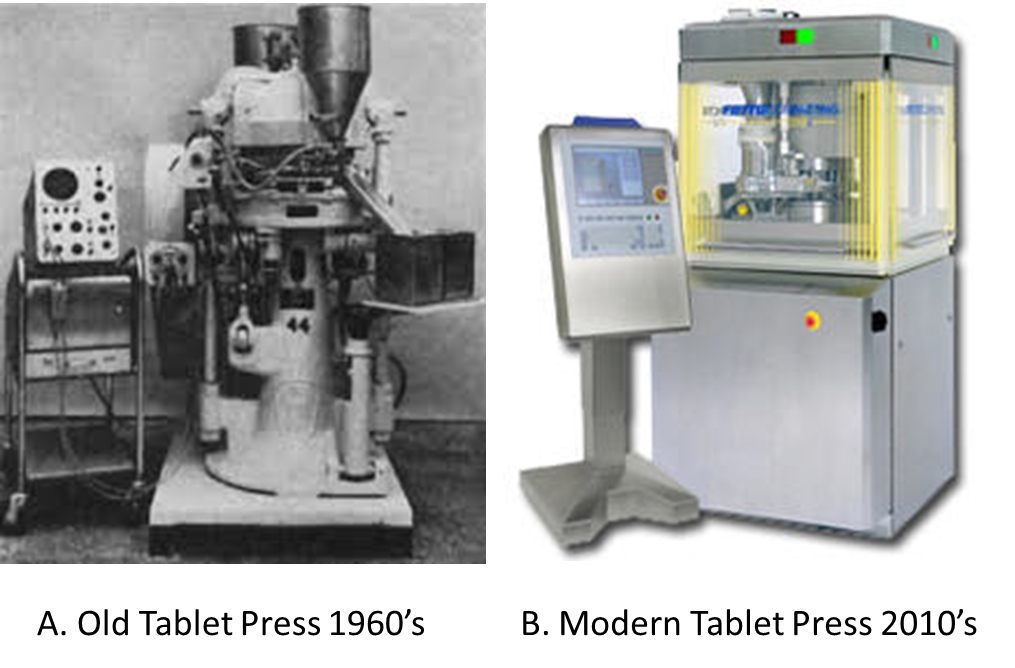
Figure 1. Comparison between an old, 1960’s tablet press machine and a recent design for the same equipment. A. A 1960’s tablet press used for research and development. Adapted from “Instrumented rotary tablet machines I. Design construction and performance as pharmaceutical research and development tools,” by Knoechel, E.L., Sperry, C.C., Ross, H.E. and Lintner, C.J. (1967). B. A typical press used in commercial product manufacturing. Adapted from “Fette America: 3200i Double Rotary Tablet Press” by Pharma Manufacturing. Retrieved from https://www.pharmamanufacturing.com/vendors/products/2010/012/
The cost of medicines in the United States has been a political balloon with practically no action at the federal government level and little advances at the state level to bring medicines affordability (Dayen, 2018, Findlay, 2019 Sept. 9th). Non-government-organizations are identifying a significant suspect in drug prices hikes as Berkeley Research Institute and pharmaceutical companies (Stampler, 2019 Feb 8th, and Vandervelde & Blalock, 2017). The Berkeley Research Report on the impact of expenditures incurred as part of the supply chain shows that 61 % of the money paid by medicines goes to others than the brand’s manufacturers (Vandervelde & Blalock, 2017). Johnson & Johnson’s CEO Alex Gorsky, on an interview with Forbes, declared that they look for drug prices transparency by publishing their net price to bring the perspective of how drug prices at the consumer level structures (Sampler, 2019 Feb 8th). With a clear culprit, the markup price of the supply chain actors, the pharmacy benefit manager (PBM), wholesalers, and distributors, no discussion on reducing the handshakes in the supply chain medicines. Another sustainability imperative of medicinal need is the issues related to supply chain vulnerabilities that arise from disruptive events associated to natural disasters, global conflicts, and pandemic events like the recent CoViD-19, which affected the supply of medicines (Jarvis, 2018, Langhauser & Parrish, 2018, and UNOC Report, 2020). The upstream and downstream supply chains are affected by disruptive events like these depriving regions of the needed medicines to treat their population.
The economies of scale drive the manufacturing decision making of generic manufacturing, forcing them to prioritize the manufacturing of high market share products over low market share, small products that serve orphan medical needs (Luzzatto, Hyry, Schieppatti, Costa, Simoens, Schaefer, Roos, Merlini, Kaariainen, Garattini, Hollak, & Remuzzi, 2018, Porath, 2018, and Reiffen & Ward, 2005). Products that generics do not want to, or cannot make, provides a compelling reason for changing the way we manufacture medicines (Archer, 2015). Three specific medicinal needs to command the convenience of manufacturing capacity in an efficient small scale and flexible medicines manufacturing system. The medical needs are low-prevalent conditions, medicines with low priority for generics capacity, broad titration medicines or precision medicines, and pediatric dosing.
- Small prevalent and generics’ neglected medicines: low-prevalent conditions, those that are suffered by a small fraction of population and are served by what is designated by the FDA as orphan drugs, rapidly become highly priced and scarce medications (Luzzatto, Hyry, Schieppatti, Costa, Simoens, Schaefer, Roos, Merlini, Kaariainen, Garattini, Hollak, & Remuzzi, 2018). The price hiking of medicines is a significant part of the generic companies’ prioritization for their capacity use, resulting in low market competitive pressure to orphan drug brand makers and their ability to sustain high prices.
- Broad titration and precision medicines: Many new and generic products are better administered when multiple doses are available and prescribed according to the patient’s needs (Schuck, Pacanowski, Kim, Madabushi & Zineh, 2019). Current manufacturing procedures used by generic manufacturers or branded products were not designed to deliver variable doses at the patient’s needs. Products that require titration or genetic markers-based products made at fixed dosages in the hope one of the dosages produced will fit the specific patient’s needs.
- Pediatric dosing: Products approved for pediatric use requires special dosing instructions that are difficult for the patient’s custodian to accurately medicate children on solid dosage forms (Institute of Medicine, 2008). Instead of breaking a tablet or dissolving them in water for dosing, a preferred type of administration would be to precisely dose capsule or tablet content to the pediatric patient’s weight.
Last but not of least importance, the most common sustainability issue, environmental impact, is another pain of pharmaceutical manufacturing today. There are three main pains in the pharmaceutical companies related to environmental impact, expired product disposition, handling of hazardous materials from operations, and solid waste management. The most important and impactful is the handling and disposal of the expired drug product (Alnahas, Yeboah, Fliedel, Abdin, & Alhareth, 2020, and Harding, 2013). The traditional method of manufacture of pharmaceutical products where the batch size is for peak demand promoting over-production. Expired production returned to wholesalers, and the products that consumers do not use, represent a significant threat to the environment. In most cases, these materials’ handling is inadequate, usually flushed through the toilets or disposed of conventional waste management facilities, resulting in a source of pollution.
Figure 2 provides a summary of the five reasons humanity needs a sustainable
All these pains may have a common relief, the design of a technology platform that enables the manufacturing of drug products, particularly solid dosage forms, in a regionally distributed system that is flexible, modular, fully automated, and portable. The innovation design described in the next sections will provide the basis for the creation of this technology platform to fulfill the needs of hospitals, pharmacies, and patients that uses generic medicines. The strategy for this innovation includes intra-organizational, extra-organizational, and customer engagement elements.
->
Figure 2. Summary of the five reasons why humanity needs to have a sustainable medicine supply.
All these pains may have a common relief, the design of a technology platform that enables the manufacturing of drug products, particularly solid dosage forms, in a regionally distributed system that is flexible, modular, fully automated, and portable. The innovation design described in the next sections will provide the basis for the creation of this technology platform to fulfill the needs of hospitals, pharmacies, and patients that uses generic medicines. The strategy for this innovation includes intra-organizational, extra-organizational, and customer engagement elements.
How are we going to reach medicines supply sustainability? The innovation process design.
Reaching these pain relief requires a focused team that considers intra-organizational, extra-organizational, and customer participation in the design and creation of the technology platform needed. The following sections provide further details of the strategy proposed to reach this innovation.
Creating an innovation project team with a strong knowledge network. As Cummings and Pletcher’s (2011) revealed in their studies, teams that use a network of expert resources in their projects have batter probability of excelling on their objectives, providing the best value of the project achievements. This innovation project team will work under a multi-functional team design in an open innovation strategy where entrepreneurs and academic resources will mingle in the creation of this technology platform. University professors and their graduate students will collaborate with industry professionals and equipment suppliers to develop this robotic manufacturing line. The industry SME’s will be designated as team mentors and will come from Puerto Rico’s industry ecosystem. These mentors are senior experts on each discipline and experience in the recent technological advances in pharmaceutical manufacturing of Continuous Manufacturing (CM) and Process Analytical Technology (PAT). The core team will have four functional groups:
- Platform Design Team: This team has the objective of designing the manufacturing platform’s mechanical systems, controls, robotic capabilities, and process design in two phases. The first phase will be a distributed continuous manufacturing line that will produce relatively higr volume products on a regional basis. The phase 1 machine will serve the generic medicines needs of a small population, 1 to 3 MM people of the most prevalent conditions in a direct distributed supply chain. This team will also design and develop in a second phase the technology platform for the manufacturing of small prevailing conditions, precision medicines, and broad titration/pediatric dosing on-demand at the point of use. The team composition will be of subject matter experts (SMEs) designated as co-principal investigators (co-PI) in the disciplines of mechanical engineering, chemical engineering, and computer engineering. An industry mentor with analog expertise will support each of the engineering professors/students’ members
- Process Analytical and In-Process Controls Design Team: This team aims to design the non-destructive chemometric analytical tools of the future manufacturing platform to create analytical systems that permit analysis without the need for sampling or testing outside the robotic unit. All product testing will occur at the manufacturing line on-site, using chemometric/parametric models for product release for patient use. This team composition will be of SMEs in chemistry, and it includes a University-based research Ph.D. in chemometrics co-PI with graduate students and an industry mentor with experience in chemometrics.
- Pharmaceutical Formulation, Process Development, Technology Transfer, and Regulatory team: This team will have the objective of designing the formulas for the selected products of the two project phases. Starting with the first six products, the formulation team will create standard formulas that should fit the formulation requirements for the line operational space. The formulation model will consider properties as flow/density and shear properties, drug load level (high and low), in a formulation strategy that minimizes the number of materials needed and provide the extended material life required for stockpiling of raw-materials for a prolonged time. This team will also design the formulas and formulation procedures for the robotic line of phase 2, will develop the technology transfer plan and the regulatory submission strategy. The team will have a professor of pharmaceutical sciences co-PI, a materials sciences co-PI, a registered pharmacist, a regulatory expert (ex FDA senior member), and an industry SME mentor.
- Supply chain transformation and sustainability team. This team will be responsible for designing the new regional and robotic point-of-use manufacturing line and its supply chain and logistics requirements. The team will collaborate with the other teams in the design for the sustainability of the technology platform, the eco-friendly design of systems components, materials handling, and the efficient use of resources to eliminate overproduction risk and waste generation. It will also design the recycling plan for the project, including means to return and re-use containers used to dispense materials during manufacturing. The team composition includes an Industrial Engineering Co-PI with graduate students’ expertise in logistics, planning, and eco-manufacturing. Two industrial SMEs will provide knowledge sharing on pharmaceutical industry environmental sciences and logistics.
Each of the sub-teams will be part of the core team, which is an overarching team. An expert in project management, previously working on a similar industry project, will mentor a graduate student that will serve as the core team lead. The industry mentor has experience leading similar innovation projects. The team will meet regularly to coordinate and finetune collaboration between sub-teams, create and maintain a task list, and ensure flawless execution of the project. The team leader will also serve as the liaison between the core team members and the project sponsors.
The team sponsorship will have three members, a senior professor or principal investigator, two customer representatives, members of a Hospital system, and a Pharmacy chain at Puerto Rico. The sponsor team will be linked to the project team with the customers through frequent interaction with the team leader and the core team. The hospital and pharmacy customer’s representatives will provide feedback to the team on the customer’s needs during the development stages. They will provide additional resources to evaluate and provide feedback on the pre-prototypes and prototypes developed during the project. The project timeline spans over five years, and the financial support for the project execution was requested through a National Science Foundation grant (NSF 20-552 Future Manufacturing Research Grant, 2020).
Figure 3 provides a diagram that depicts the project team composition and the interactions between them.
In summary, the project team will execute this innovation plan in two phases. Phase 1 will provide the benefits of regionalized manufacture of generics, allowing for adequate stockpiling of materials with an extended expiration and adequately addressing the needs for medicines availability in a supply chain disruption event. The design of this phase 1 line is amenable for installation in remote locations to serve deprived populations or any place where medicines need to be, even at Mars. The phase 2 robotic micro-line will provide additional benefits associated with the duly required precision or personalized medicines. It will open a new way to deliver old generic doses with broad titration needs and future branded orphan drug products. Figure 4 shows a roadmap of this innovation project.
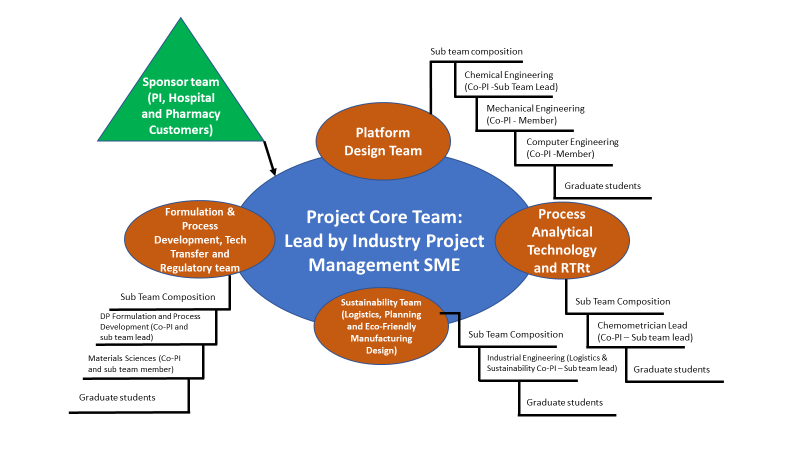
Figure 3. Innovation project team composition and sponsorship.
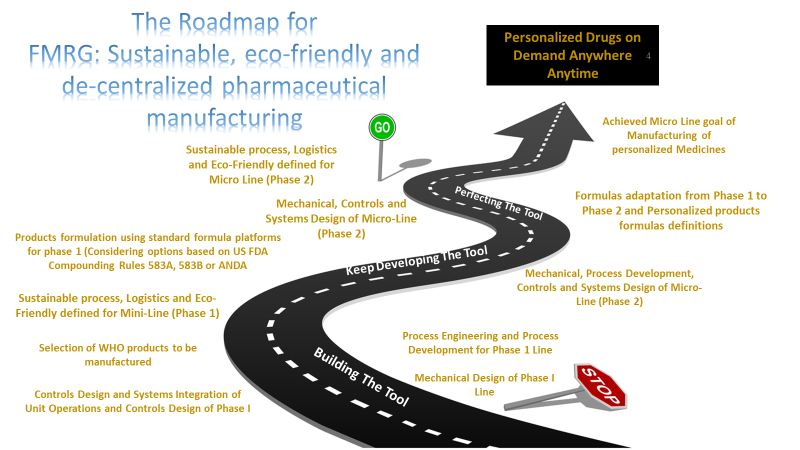
Figure 4. Overall project road map showing the two phases of the project toward the robotic micro-manufacturing line
The innovation project: A project design based on a cluster of innovation and open innovation. Part of the success of this significant innovation project will require an orchestration of a significant number of industry providers, academic experts, hospital & pharmacy providers, customers, entrepreneurs, and the participation of industry experts and regulators. A cluster of innovation, and possibly a model based on open innovation can provide the necessary resources that can take this project into a successful completion (Tuulenmäki & Välikangas, 2011, and Engel & del-Palacio, 2011). The New Jersey area is a hub or cluster of innovation for the biopharmaceutical sector (Bio NJ, 2018). It is composed of several major biopharmaceutical companies, equipment, materials, and services providers, as well as academic institutions with active collaboration with the bio sector. Among the institutions that have traditionally compose the academic collaborators are Rutgers University, NJIT, and Princeton University. One example of an open collaboration between equipment suppliers, academic partners, and industry members for the development of Continuous Manufacturing and Real-Time Release technologies for the manufacture of solids dosage forms. The National Science Foundation-sponsored, an Engineering Research Consortia of Industry and Academia where Rutgers University, Purdue University, NJIT, and the University of Puerto Rico Mayaguez participated in the development of these technologies for sponsors like Johnson & Johnson. These learnings help Janssen Supply Chain develop a solution to the elusive JIT full application in the pharmaceutical processing environment, with the participation and sponsorship of the FDA (NSF, 2006).
This innovation process design will leverage the relationship and knowledge base gain during the NSF sponsored ERC-SOPS consortia and expanded on it in a similar open collaboration approach. This project plan, as described in the previous section, will relly on the collaboration with academia, industry members and equipment, materials, and systems suppliers at the NJ COI and Puerto Rico. Table 1 shows the proposed cluster companies, academic centers, industry members, entrepreneurs’ companies and customers called for collaboration
Involving the customer in the innovation design process. Customer involvement in creating innovation is of extreme importance (Sandmeier, Morrison & Gassmann, 2010). Sandmeier et al. (2010) mention in their article the closing statement that, translating customer involvement in the regulated industry must be done carefully, it is though something possible in this case. Since our objective is to create a system that improves medicine availability, it is entirely reasonable to use the customer to co-create this product. This is why we decided to include the customer in the sponsor’s team. The sponsor team includes the principal investigator, the customers including a hospital and pharmacy delegates, and the project team lead. The customers will be exposed to the products from the phase 1 regional manufacturing line and later in phase 2 those that will secure our technology platform to compound their generic products. Our plan provides for an iterative process of design, experimentation, prototyping, customer feedback, analysis, and when needed, re-design, and repeat the process. This approach permits the continued and reiterative examination of our product hardware and software components by our customers to ensure that what they need is what they get. Figure 5 shows a diagram depicting the product development process and the customer feedback process.
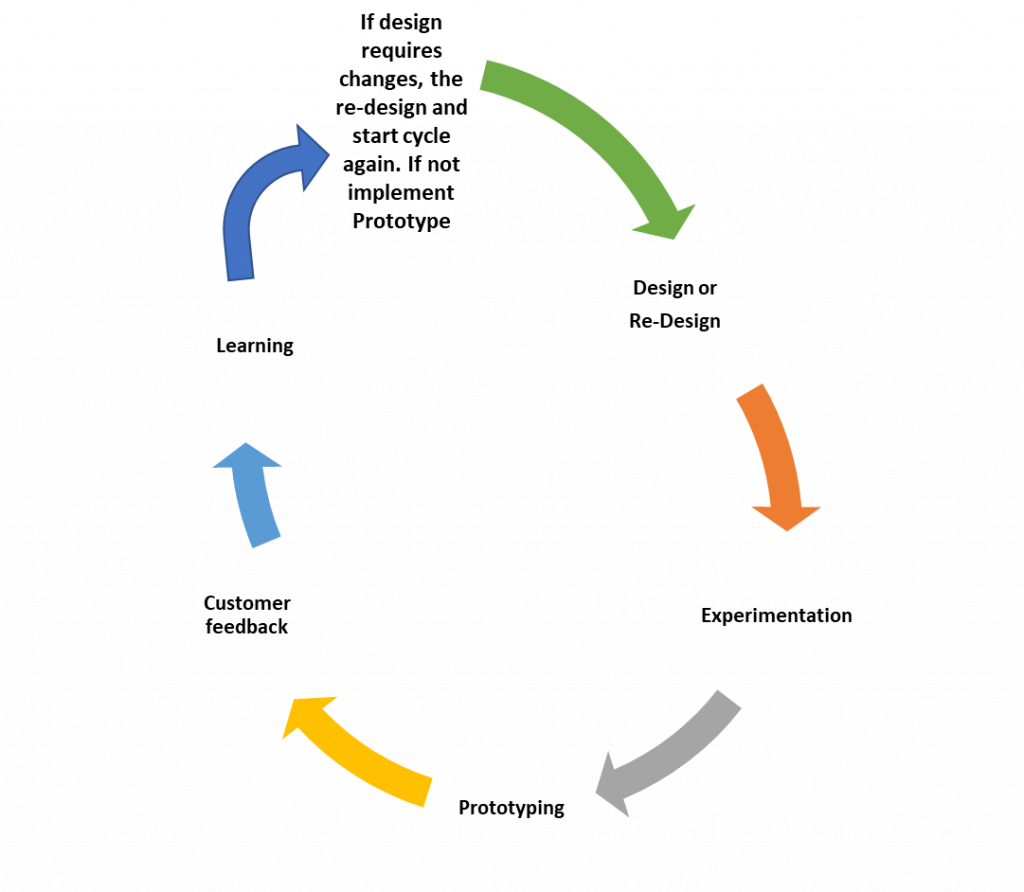
Figure 5. Diagram of the customer involvement iterative process.
Conclusion
There is an imperative need for the transformation of medicines supply, allowing for improved sustainability of drug manufacturing in many angles. This innovation process design provides a guideline on how to ensure a successful innovation in the production of medicines that considers intra-organizational aspects, clusters of innovation with open innovation, and customer involvement in the design of the innovative solution. A successful grant approval by NSF depends not only on the scientific merit of the proposal; it also needs to prove its viability for a future commercial deployment. The innovation design provided in this document permits a project’s focus on proven methodologies that showed to be successful for many firms on the development and implementation of new products.
References
Alnahas F, Yeboah P, Fliedel L, Abdin, A.Y., and Alhareth, K. (2020). Expired Medication: Societal, Regulatory and Ethical Aspects of a Wasted Opportunity. Int J Env Res Public Health 17 (3): 787 Retrieved from https://www.mdpi.com/1660-4601/17/3/787
Archer, R. (2015). Precision medicine; imprecise manufacturing and supply. The Medicine Maker. Retrieved from https://themedicinemaker.com/manufacture/precision-medicine-imprecise-manufacturing-and-supply
Bio New Jersey (2018). The New Jersey biopharmaceutical industry: A prescription to growth. Bio NJ. Retrieved from https://bionj.org/the-new-jersey-biopharma-industry-a-prescription-for-growth
Chatterjee, S. (2012). FDA perspective on continuous manufacturing. IFPAC Annual Meeting, Baltimore, MD. Retrieved from https://www.fda.gov/media/85366/download
Cooper, R. G., Edgett, S. J., & Kleinschmidt, E. J. (2002). Optimizing the stage-gate process: What best-practice companies do-I. Research Technology Management, 45(5), 21-27. Retrieved from http://library.capella.edu/login?qurl=https%3A%2F%2Fsearch.proquest. com%2Fdocview%2F213806181%3Facc
Cummings, J., and Pletcher, C. (2011). Why project networks beat project teams. MIT Sloan Management Review, 52(3), 75-80. Retrieved from http://library.capella.edu/login?qur l=https%3A%2F%2Fsearch.proquest.com%2Fdocview%2F861418971%3Faccounti
Dayen, D. (2018). Elizabeth Warren plan would allow the government to manufacture its own generic drugs. The Intercept. Retrieved from https://theintercept.com/2018/12/18/elizabeth-warren-generic-drugs-bill/
Engel, J.S., and del-Palacio, I. (2011). Global clusters of innovation: The case of Israel and Silicone Valley. California Management Review, 53 (2), 27-49. https://doi-org.library.capella.edu/10.1525/cmr.2011. 53.2.27
Findlay, S. (2019). States pass record number of laws to reel in drug prices. Kaiser Health News. Retrieved from https://khn.org/news/states-pass-record-number-of-laws-to-reel-in-drug-prices/
Gottlieb, S. and Woodcock, J. (2019). FDA is advancing new efforts to address drug shortages. FDA web page blog, retrieved from https://www.fda.gov/NewsEvents/Newsroom/ FDAVoices/ucm626108.htm
Harding, C. (2013). The environmental impact of the pharmaceutical industry. US Green Technology. Retrieved from https://usgreentechnology.com/the-environmental-impact-of-the-pharmaceutical-industry/
Hausner, D.B. and Moore, C.M.V (2018). Continuous manufacturing current status. Pharmaceutical Engineering. Retrieved from https://ispe.org/pharmaceutical-engineering/may-june-2018/continuous-manufacturing-current-status#
Institute of Medicine (2008) Forum on Drug Discovery, Development, and Translation. Addressing the Barriers to Paediatric Drug Development: Workshop Summary. Washington (DC): National Academies Press (US). Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK3995/
Jarvis L. (2018). Hurricane Maria’s lessons for the drug industry. Chemical & Engineering News. American Chemical Society. Retrieved from https://cen.acs.org/pharmaceuticals/biologics/Hurricane-Marias-lessons-drug-industry/96/i37.
Knoechel, E.L., Sperry, C.C., Ross, H.E and Lintner, C.J. (1967). Instrumented rotary tablet machines I. Design construction and performance as pharmaceutical research and development tools. Journal of Pharmaceutical Sciences 56 (1) 109-115. DOI: https://doi.org/10.1002/jps.2600560122
Langhauser K and Parrish M. (2018) Puerto Rico pharma: battered but unbroken. Pharmaceutical Manufacturing. Retrieved from https://www.pharmamanufacturing.com/articles/2018/puerto-rico-pharma-battered-but-unbroken/
Luzzatto, L., Hyry, H., Schieppatti, A., Costa, E., Simoens, S., Schaefer, F., Roos, J.C.P, Merlini, G., Kaariainen, H., Garattini, S., Hollak, C.E., and Remuzzi, G. (2018). Outrageous prices of orphan drugs: a call for collaboration. The Lancet 392 (10149) 791- 794. Retrieved from https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31069-9/fulltext
National Science Foundation (2006). NSF awards $75.3 Million for five new engineering research centers. Retrieved from, https://www.nsf.gov/news/news_summ.jsp?cntn_id= 107939
National Science Foundation (2020). Call for proposal for Future Manufacturing Research Grant, NSF-20-522. National Science Foundation. Retrieved from https://www.nsf.gov/pubs /2020/nsf20552/nsf20552.htm
Pharmaceutical Technology Editors (2016). FDA approved tablet production on Janssen continuous manufacturing line. PharmTech.com. Retrieved from http://www.pharmtech .com/fda-approves-tablet-production-janssen-continuous-manufacturing-line
Porah, D. (2018). Size and dynamics of order-of-entry effects in pharmaceutical markets. International Journal of Market Research 60 (1) 50-66. DOI: 10.1177/1470785317744669 journals.sagepub.com/home/mre
Sandmeier, P., Morrison, P.D., and Gassmann, O. (2010). Integrating Customers in Product Innovation: Lessons from Industrial Development Contractors and In-House Contractors in Rapidly Changing Customer Markets. Creativity & Innovation Management, volume 19, issue 2, pages 89–106. Retrieved from http://web.b.ebscohost.com.library.capella.edu/ehost/pdfviewer/pdfviewer?vid=1&sid=2dfa07ff-9c5c-4a9f-9b39-75411aa73e67%40sessionmgr103
Stampler, L. (2019, Feb.8th). Johnson & Johnson will be the first drug company to put prescription medication prices in TV ads. Forbes. Retrieved from https://fortune.com/2019/02/08/johnson-and-johnson-drug-price-tv-ads/
Tuulenmäki, A., and Välikangas, L. (2011). The art of rapid, hands-on execution innovation. Strategy & Leadership, 39(2), 28-35. doi:http://dx.doi.org.library. capella.edu/10.1108/10878571111114446
Vandervelde, A. and Blalock, E. (2017). The pharmaceutical industry supply chain: gross drug expenditures realized by stakeholders. Berkeley Research Group Report. Retrieved from https://www.thinkbrg.com/newsroom-publications-pharma-supply-chain.html#
